I. Introduction
Our body constantly exchanges materials with external surroundings via the skin, which is mainly mediated by the skin barrier including the stratum corneum(SC) and extracellular lipids1). Recently, skin barrier dysfunction has emerged as a major pathogenesis in atopic dermatitis(AD). Skin moisture is excessively evaporated through the damaged skin barrier, causing dehydrated skin to provoke pruritus, and the patient aggravates AD by scratching the skin2). On the other hand, the damaged skin barrier becomes an opened gate for external allergens, which also aggravate AD by promoting systemic inflammation3). Thus, a solid skin barrier is necessary to regulate AD, but it paradoxically impedes percutaneous absorption1).
AD manifests diverse symptoms in each patient. Skin xerosis is the most frequently observed symptom among minor manifestations of AD4). Considering the damaged barrier, the SC in atopic xerosis not only exhibits insufficient hydration but also demonstrates enhanced transepidermal water loss(TEWL), pH, and turnover rate5).
Clinically, topical medications are routinely prescribed for skin diseases. In spite of its many advantages in respect of patient compliance and drug metabolism6,7), this method of transdermal drug delivery has low efficiency due to blockage by the skin barrier8). A microneedle(MN) patch was first invented in 1976 to overcome such limitations9). The skin barrier is penetrated with the MN, and the patch enhances drug absorption compared with SC delivery10). Due to these advantages, MN patches have been spotlighted not only in pharmaceuticals but also in cosmetics and vaccinations11,12).
Prior animal studies have demonstrated potential applications of MN patch to skin inflammation13,14). Two clinical trials suggested that a biodegradable MN patch may be useful for improving prurigo nodularis and psoriatic plaques15,16). However, this study used an MN patch for xerosis in AD patients. To establish clinical evidence for drug delivery medical devices in AD, we examined the efficacy and safety of a biodegradable hyaluronic acid MN(BHMN) patch for atopic xerosis. Thus, the use of complex treatment with a BHMN patch and an aroma cream(BHMN patch complex therapy) was compared with that of aroma cream alone.
II. Materials and Methods
This study was originally a prototype of our following study on applying the BHMN patch to AD. Thus, the methods used in this study are similar to the later study17).
In a single Korean medicine hospital, this study was conducted as an investigator-initiated, assessor-blinded, split-body, randomized controlled parallel trial. To avoid bias, the outcomes investigator was permitted to contact the subjects only during their examination.
We intended to recruit 20 AD patients and xerosis based on the results from a clinical study for prurigo nodularis15). In that study, the intergroup difference, significance level, and power were set as 8.3±11.8%, 5%, and 80%, respectively. We substituted the aforementioned variables according to the following formula:
where “npair”, “zα/2”, “zβ”, “σ”, and “difference” represent the sample size, Z-score for the significance level, Z-score for the power, standard deviation, and intergroup difference, respectively.
Considering a 20% dropout rate, the calculated sample size was approximately 20. Subjects were recruited via poster advertisements at the Naju Dongshin University Korean Medicine Hospital (NDSUKH). The inclusion and exclusion criteria are presented below. Patients with any of the exclusion criteria were ineligible.
-
① Those aged between 19-60.
-
② Those who have AD and dry skin. AD was diagnosed with the Hanifin and Rajka criteria18).
-
③ Those who have two comparable lesions with atopic xerosis on their extremities.
-
④ Those who willingly agreed to informed consent.
-
① Those who are receiving intensive medication treatment, including antihistamines or corticosteroids.
-
② Those who took medicine including antihistamines, antibiotics, corticosteroids, or immunosuppressants, applied local corticosteroids, or received systemic photochemotherapy within four weeks prior to the study beginning.
-
③ Those who have systemic infections or are receiving systemic antibiotic treatment.
-
④ Those who have other severe dermatoses besides AD.
-
⑤ Those who were treated with interferon medications.
-
⑥ Those who have hepatic illnesses such as cirrhosis or liver carcinoma.
-
⑦ Those who have renal illnesses such as renal failure or nephrotic syndrome.
-
⑧ Those who have serious acute cardiovascular illnesses such as heart failure, myocardial infarction, or stroke.
-
⑨ Those who took antipsychotics within three months before the screening.
-
⑩ Those who are sensitive to natural materials.
-
⑪ Those who are sensitive to adhesives.
-
⑫ Pregnant or breastfeeding females.
-
⑬ Those who have severe exudation or erosion.
-
⑭ Those who are determined to be unsuitable by the investigators.
All subjects were briefed on this study before its beginning, and written consent was acquired. The subjects were compensated for their participation with a prescribed transportation fee when the trial ended. This study obeyed the Declaration of Helsinki and got approval from the Institutional Review Board of Naju Dongshin University Korean Medicine Hospital(Approval No. NJ-IRB-006). This study was registered with the Clinical Research Information Service, Republic of Korea(Registration No. KCT0005942, available from: https://cris.nih.go.kr/cris/search/detailSearch.do/18808).
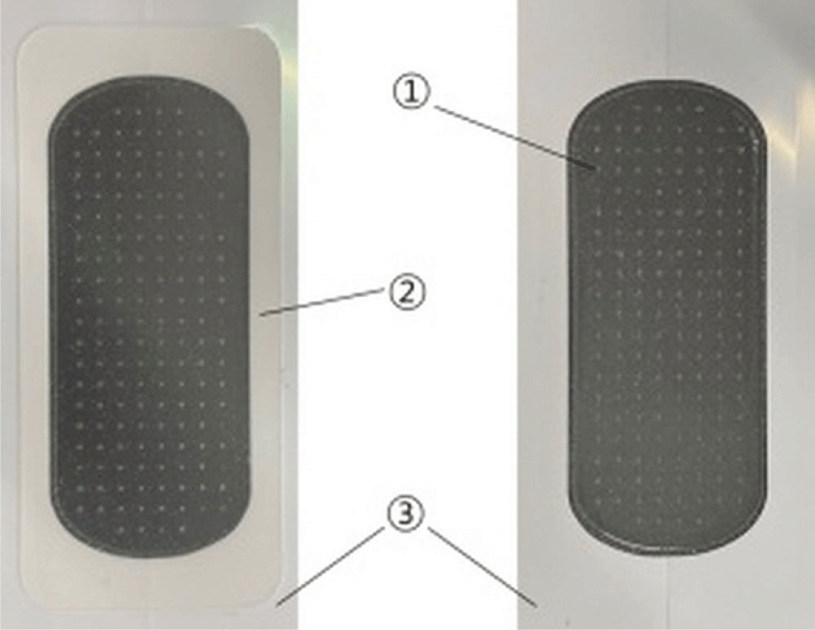
We covered the experimental side of the upper extremities where the aroma cream was applied with a sterile BHMN patch(RMD-2.5KM, Raphas Co. Ltd., Seoul, Republic of Korea). This patch comprised medical hyaluronic acid MNs on a hydrocolloid film(Table 1, Fig. 1).
| Model | RMD-2.5KM |
|---|---|
| Needle length(㎛) | 250 |
| Width(including release paper, mm) | 66 |
| (excluding release paper, mm) | 60 |
| Length(including release paper, mm) | 31 |
| (excluding release paper, mm) | 27 |
| Weight(㎎) | 810±81 |
An aroma cream dispensed by the Korean medicine ophthalmology, otolaryngology & dermatology clinic of NDSUKH was used. The cream was made up of a base lotion and two kinds of aroma oil. The major ingredients of the lotion(Korean Oriental Medicine Naturopathy Institute, Seoul, Republic of Korea) were ceramide, aloe vera, squalane, and herbal extracts, including Glycyrrhizae Radix, Coptidis Rhizoma, green tea, and Scutellariae Radix. Tea tree oil and lavender oil(four drops each) were combined with 30g of the lotion. On visit 1, 10g of the aroma cream was provided to all subjects.
This study was carried out between December 28th, 2020, and January 22nd, 2021. The study phases are composed of the screening and the treatment. The applicants were screened two weeks before the intervention. They visited once at the beginning and once at the end of the treatment. Thus, eligible subjects attended three visits throughout the study(Table 2).
Following the subjects’ provision of written consent, the investigators checked their vital signs, sociodemographic information, present illness, past history within six months, and medication history. Their vital signs and medication history were repeatedly evaluated on visits 1 and 2. Subjects were selected according to the screening criteria. At every visit, the investigators informed the subjects about the following visit.
Visit 1 was scheduled two weeks after the screening visit. On visit 1, the experimental side of each subject was randomly determined by drawing lots for either the left or right extremity. Following randomization, the investigators captured photographs of the parts of the skin where the trial was conducted and examined the subjects using the Hanifin and Rajka criteria18), Local Scoring AD(L-SCORAD) index19,20), Investigator’s Global Assessment(IGA) scale19,21,22), Scoring AD(SCORAD) index23), Dermatology Life Quality Index(DLQI)24), pruritus and dryness visual analog scale(VAS)25), skin hydration, and TEWL19). The subjects were provided with the BHMN patches and aroma cream after their examination and were given instructions on their correct usage.
Visit 2 was scheduled two weeks after visit 1. On visit 2, subjects were requested to return the packages of used BHMN patches and remnants of aroma cream to evaluate their compliance. Moreover, the investigators captured photographs of their skin and measured the outcomes. Adverse events(AEs) and subjects’ satisfaction were also investigated.
Each intervention was treated to the same upper extremity of the same subject. Namely, both aroma cream and a BHMN patch were applied to the left limb if the left side was allocated in the experimental group(BPG), and aroma cream alone was applied to the right side(CtrlG). For the experimental area, a pair of similar lesions on the forearms were mainly selected.
The subjects applied aroma cream to both sides and attached the BHMN patch only to the experimental side daily for two weeks. The BHMN patch was attached for eight hours, i.e., before going to sleep until the next morning. The investigators provided the following instructions for patch attachment:
-
① Clean the experimental lesion before use. Do not apply any unauthorized cosmetics or topical agents together on the experimental area.
-
② Apply a small amount of the aroma cream, as much as a fingertip unit, to the experimental area. When applying the aroma cream, the medication should not be applied to the adhesive area, which is located at the margin of BHMN patch and does not have MNs.
-
③ Remove the release paper and attach the patch on the aroma cream. While handling, the MNs on the BHMN patch should not be contacted. The patch should be vertically attached on the skin.
-
④ Press the patch evenly for 10 seconds.
On the other side of the experimental limb, aroma cream alone was applied for two weeks.
The L-SCORAD index was the primary outcome. Other outcomes, including the IGA scale score, SCORAD index, DLQI, pruritus and dryness VAS scores, skin hydration, TEWL, and compliance and safety assessment, were the secondary outcomes.
To inclusively examine the symptoms of a dry lesion, the L-SCORAD index was adopted as an outcome measure. The L-SCORAD index is a modified version of the SCORAD index designed to examine local lesions. It grades six objective AD symptoms(redness, edema/papule, excoriations, lichenification, exudation/crusts, and dryness) from 0 to 3 points according to symptom severity. The maximum total score is 1820). Subjects were instructed to score both test lesions in accordance with the following criteria: 0=no symptoms, 1=mild, 2=moderate, and 3=severe.
The IGA scale provides a simple assessment of AD severity. Thus, it has been used as a general outcome in clinical trials for AD. However, its criteria and definitions across studies have been variable21,22). This study used a 6-grade IGA scale classified by lesion severity(Table 3).
The SCORAD index has been a widely used outcome measure for AD since its development by the European Task Force on Atopic Dermatitis in 199323,26). It consists of an examination of eczema size, severity, and subjective symptoms of the patient. Lesion extent was determined by scoring the body parts affected by AD according to the rule of nines. We assessed severity by summing the scores for six objective symptoms including redness(acute)/pigmentation(chronic), edema/papule, exudation/crusts, excoriation, lichenification, and dryness. Moreover, a patient-subjective assessment was conducted by scoring pruritus and sleep disorders related to the quality of life(QoL)23).
To survey the QoL of the subjects, we used the Korean version of the DLQI24). The DLQI is the first reliable evaluation method for QoL and has been adopted in many studies. Moreover, it includes only a few questions; therefore, QoL is simple to evaluate27). These questions survey the symptoms, feelings, daily lives, leisure activities, job performance, personal relations, and treatment of subjects. The response to each question is rated as follows: 0, “not relevant” or “not at all;” 1, “a little;” 2, “a lot;” and 3, “very much.”
The total score ranges from 0 to 30, with a higher score suggesting the adverse effects of AD on QoL24). This study received a free license for the DLQI from the School of Medicine, Cardiff University(License ID : CUQoL3488).
A VAS was used to assess the severity of itch and xerosis. A straight line marked from 0 to 10 was presented to the subjects. On the pruritus VAS, a score of 0 meant no pruritus at all, while 10 meant unbearable pruritus. On the dryness VAS, a score of 0 meant no dryness at all, and 10 meant unbearable dryness. To help better understanding, the faces pain scale was also suggested below the VAS line. Six faces indicating no, little, moderate, severe, very severe, and the worst possible symptoms were denoted by 0, 2, 4, 6, 8, and 10, respectively. The subjects ranked the intensity of the symptoms.
A Corneometer CM825(Courage + Khazaka electronic GmbH, Köln, Germany) was used to measure skin hydration. The instrument presents skin moisture by measuring dermal capacitance. Evaluations were carried out after the subjects were tranquilized for 30 minutes in conditions of 20-25°C and 40-60%. An investigator put a 10㎜-diameter probe on the lesion, exerting a pressure of 1-1.5N. The measurement was carried out five times, and after excluding the minimum and maximum values, the mean of the remaining three values was calculated. The result was suggested as a score ranging from 0 to 130. Considering the positive correlation between moisture and skin capacitance, higher values suggest greater skin hydration. Values of <30, 30-45, and >45 respectively corresponded to extremely dehydrated, dehydrated, and fully moisturized skin in the extremities.
The integrity of the skin is indicated by TEWL. Skin moisture evaporation is proportional to TEWL. A Tewameter TM300(Courage + Khazaka electronic GmbH, Köln, Germany) was used to measure TEWL. The measurements were carried out after the subjects were tranquilized for 30 minutes under the same surroundings as stated above. An investigator put a probe on the lesion for 30-40 seconds, and the settled value was recorded as the measured value. The measurement was carried out thrice, and the mean value was analyzed. Values of 0-10, 10-15, 15-25, 25-30, and >30g/h/m2 respectively corresponded to extremely healthy, healthy, normal, strained skin, and critical conditions.
To investigate compliance, the investigators instructed the subjects to regularly upload photographs of the BHMN patch attached to the skin to a social media application(Kakao for Business, Kakao Corp., Republic of Korea) designed for this study. Moreover, the subjects were requested to return the BHMN patch packages and remnants of aroma cream on visit 2.
AEs were monitored every visit from the beginning of the study until one week following visit 2. Temporary redness, edema, irritation, and eczema resulting from an adhesive allergy were anticipated as BHMN patch-related AEs. Upon recording an AE, the trial of the subject was suspended.
All outcome measures were analyzed using statistical software(SPSS Statistics 21, IBM, NY, USA). A p-value of <0.05 was judged to be statistically remarkable. The results were analyzed in accordance with an intention-to-treat design. For the missing values, the last-observation-carried-forward was used. The Mann-Whitney test was adopted to compare the two groups. The Wilcoxon signed-rank test was adopted to compare the results before and after treatment following the two weeks of intervention.
III. Results
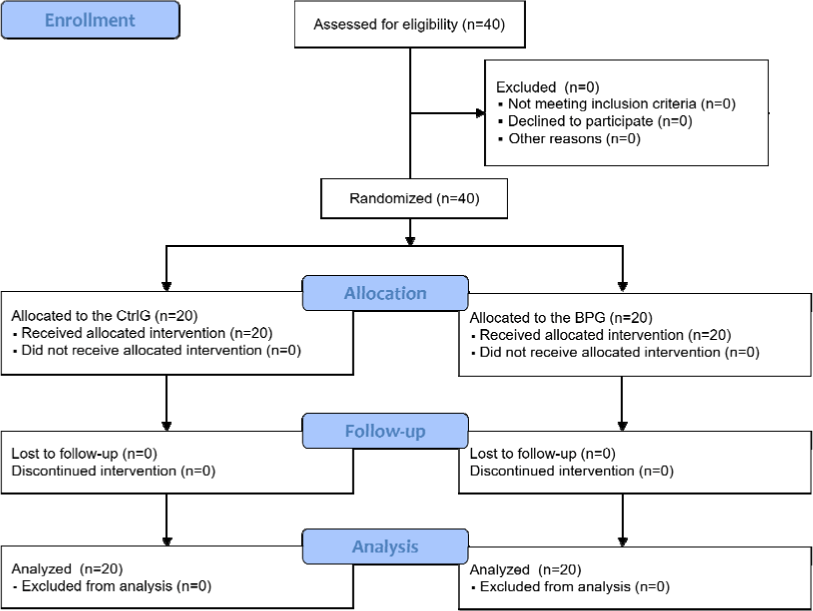
All recruited 20 subjects finished this trial, and a total of 40 lesions were finally analyzed(Fig. 2). The sex ratio was 9:11. The age range was 20-40 years, and the average age was 26.7±5.55 years. Only two subjects were smokers, and 16 consumed alcohol. Fifteen patients did not have general past history within the six months prior to study initiation, and 14 patients had not taken any medication during the same period(Table 4).
The L-SCORAD index substantially declined in the BPG compared with the CtrlG. On the first visit, the index of the two groups was equal. On the next visit, both groups demonstrated a remarkable reduction relative to the baseline value. However, the index of the BPG remarkably declined compared with that of the CtrlG(Table 5).
| PG(n=20, mean±SD) | CtrlG(n=20, mean±SD) | p-value* | |
|---|---|---|---|
| Visit 1 | 5.2±1.50 | 5.2±1.50 | 1.000 |
| Visit 2 | 1.4±1.06 | 3.0±1.32 | |
| Difference | 3.9±1.15 | 2.3±1.13 | 0.000*** |
| p-value** | 0.000*** | 0.000*** |
The IGA scale grade remarkably improved after two weeks in both groups. However, any differences were not observed between them. On the first visit, the IGA scale grade in both groups was the same. After two weeks, the grade in both groups equally declined(Table 6).
The SCORAD index and DLQI remarkably declined after two weeks(Table 7). Thus, the QoL of the subjects appeared to improve after applying the BHMN patch.
| SCORAD index(n=20, mean±SD) | DLQI(n=20, mean±SD) | |
|---|---|---|
| Visit 1 | 28.1±3.10 | 4.3±3.98 |
| Visit 2 | 20.4±4.64 | 2.2±2.57 |
| Difference | 7.7±3.15 | 2.2±2.13 |
| p-value* | 0.002** | 0.002** |
The pruritus VAS score of each group remarkably declined after two weeks. However, no differences were observed in intergroup comparisons. On the first visit, the scores of the two groups were similar. The score of the BPG declined more than that of the CtrlG on the next visit(Table 6).
The dryness VAS score of each group remarkably improved after two weeks. However, a greater reduction was observed in the BPG. On the first visit, the score was similar between the two groups. The score of the BPG declined more than that of the CtrlG on the next visit(Table 6).
Skin hydration remarkably shifted in all comparisons. The skin of the BPG was remarkably moisturized compared with that of the CtrlG. On the first visit, the values of the two groups were alike, corresponding to extremely dry skin. After two weeks, the value of the CtrlG was consistent with dry skin. The value of the BPG corresponded to sufficiently moisturized skin(Table 6).
Low TEWL indicates a healthy skin barrier. TEWL remarkably declined in both groups after two weeks. However, there was no remarkable difference between the two groups. On the first visit, the TEWL values, which were statistically unremarkable, were similar between the two groups. After two weeks, the decline in the CtrlG was larger than that in the BPG(Table 6).
IV. Discussion
This study adopted a single-blind design since both interventions were simultaneously applied to the same subject. Consequently, the subjects and investigators both knew where the interventions were applied. To preserve the single-blind design, the investigator who examined the outcomes and the clinical research coordinator were separate. The outcomes investigator was allowed to meet the subjects only during the examination time. Moreover, separate report forms that did not contain personal information were provided for the outcomes investigator.
Transdermal drug delivery, which includes the use of a topical cream or patch, has many benefits, including high compliance, avoidance of liver metabolism, a large surface skin area to absorb the drug, and immediate end of drug administration6,7). However, few medications successfully deliver therapeutic agents into the skin because of the SC, the key component in the skin barrier8). For example, only a small dose(a maximum of 0.3%) of active compounds included in a topical medication can be absorbed into the skin; most active compounds are uselessly wasted10).
In 1976, an MN patch was designed to complement the limitations of conventional transdermal drug delivery systems9). MNs arranged on an MN patch temporarily disrupt the skin to penetrate the SC28). The drug can then sufficiently reach the upper tissue of the skin, where it can enter the systemic circulation and ultimately exhibit the intended therapeutic response at the target area28). Consequently, MN patches enhance drug use efficiency compared with percutaneous delivery via the SC13). Similarly, an MN therapy system roller raises percutaneous absorptance by as much as 1,000 times29).
Recently, the MN patch technology has been vastly expanding its applied field, including facial wrinkle care11), pharmaceutical purpose, vaccination12), genetic engineering13), immunotherapy14), pain control30), and skin diseases15,16,31). For dermatological use, the MN patch is a promising novel therapy for skin cancer, psoriasis, AD, skin infections, and viral warts31). However, human clinical trials on applying BHMN patch to skin diseases are rare. The representative prior studies were for prurigo nodularis and psoriasis. In patients with prurigo nodularis, combination treatment with a biodegradable MN patch and mometasone furoate 0.1% cream much improved the area and height of skin lesions and the pruritic VAS score after four weeks of treatment, compared to only the topical medication15). In psoriatic patients resistant to vitamin D derivatives-corticosteroids complex ointment, a combined treatment with a BHMN patch and the same ointment for a week improved their symptoms and plaques16).
Pruritus and eczema are representative symptoms of AD. In addition, various symptoms including redness, papules, vesicles, erosions, exudation, or crusts are manifested on the skin of AD patients. Although the skin looks undamaged, dryness, coarseness, or scales can be observed5,32). The skin barrier is impaired by abnormal inflammation arising from the SC, which leads to the aggravation of atopic xerosis. This is supported by the reduction in hydration, ceramides, and amino acids, increased pH, turnover rate, and TEWL, smaller corneocytes, and excessive sensitivity to external biochemical irritation5). Thus, it is recommended to apply moisturizer as the standard treatment for AD33).
Moisturizer helps to improve xerosis and to prevent inflammation exacerbated by recurrent scratching by restoring intercellular lipid barrier function32,34). Although it is simple, applying moisturizer is effective for AD. Mild AD can be sufficiently improved by applying moisturizer alone32,35). Even for severe AD, which requires complex treatment including corticosteroids, applying moisturizer can reduce topical corticosteroid use36).
In this study, applying aroma cream was a common intervention for each group. The aroma cream has been prepared and provided for AD at our clinic. The added aroma oils to the cream were tea tree and lavender oil. Although the former is usually reported for its anti-infective activity36), it has also been reported to soothe skin inflammation37). The latter is mainly used as an essential oil for AD, which helps to calm itch by inhibiting immunoglobulin E production38). In addition, the main constituents of the base lotion are specialized for skin moisturizer and soothing AD31,40-2).
The SCORAD index and DLQI indicated a likely improvement in QoL. The DLQI surveys the influence of skin diseases on patients during the prior week. In contrast, the SCORAD index examines not only the skin lesions but also the severity of sleep disturbances and pruritus. As abovementioned, the VAS scores remarkably declined after two weeks, which may have influenced the improvement in the two outcomes.
The BPG showed a bigger improvement in the dryness VAS scores and skin hydration. The decline in the dryness VAS score may have been affected by enhanced skin hydration. Although there was no remarkable difference in the intergroup comparison, enhanced skin hydration may have improved the pruritus VAS score. The finding of enhanced skin moisture is consistent with those reported in prior studies43,44). Hyaluronic acid was used as the major ingredient of the MN since it is a natural glycosaminoglycan that can be used as a moisturizer due to its strong water-binding ability45). Thus, such improvements may have resulted from the moisturizing effect of the hyaluronic acid MNs. Furthermore, hyaluronic acid may have contributed to the safety of the BHMN patch. Hyaluronic acid itself has been shown to be safe when injected into the body46,47). In addition, MN patches fabricated with hyaluronic acid have not provoked skin abnormalities in prior studies11,15,47).
Despite the remarkable intragroup difference, the BHMN patch did not offer greater improvements in other outcomes. The CtrlG showed less TEWL than the BPG, indicating a more solid skin barrier. However, prior studies48,49) have suggested that this phenomenon is temporary, due to the perforation of the BHMN patch. Thus, the enhanced TEWL of the BPG does not indicate abnormal damage to the skin barrier. Furthermore, the intergroup difference on visit 2 may become similarly balanced with time.
In conclusion, the BHMN patch complex therapy for two weeks led to greater improvement in xerosis in AD patients than aroma cream alone. These results may be due to the ability of BHMN patches to aid in the skin absorption of aroma cream. In addition, MNs consisting of hyaluronic acid may have contributed to the moisturizing activity of the BHMN patch. The BHMN patch was safe since no related AEs were reported during the study period.
The BHMN patch complex therapy did not remarkably affect several outcome measures including the IGA scale score, pruritus VAS score, and TEWL. Such limitations may have resulted from the short study period and small sample size. Although we took photographs of test lesions from all subjects on visits 1 and 2, the absence of notable photographs presenting remarkable changes in the skin was also a shortcoming in this study. The insignificant external changes present in the photographs may be because the overall severity of AD in the subjects was mild. If we had taken detailed photographs of the lesions, our results could have been more reliable. Above all, the absence of a proper control intervention is the weakest point of our study. Designing a proper control intervention, including the preparation of a needleless patch is necessary to further studies.
Our study found a new insight into the use of a BHMN patch for atopic xerosis. The BHMN patch complex therapy is a safe and useful alternative for xerosis in AD and can potentially be used in other skin diseases.
V. Conclusions
The BHMN patch complex therapy remarkably improved the L-SCORAD index compared with aroma cream alone. The BHMN patch complex therapy also remarkably improved the dryness VAS score compared with aroma cream alone. However, the BHMN patch complex therapy did not show superiority over the control treatment in terms of the pruritus VAS score. The BHMN patch complex therapy remarkably raised skin hydration compared with the control treatment. In addition, the SCORAD index and DLQI also remarkably improved, reflecting improvement in patient QoL. Although there was no difference in the effect on some secondary outcomes, the BHMN patch complex therapy notably improved AD compared with the topical application alone without producing any serious or unsuspected AEs.






