1. Introduction
Inflammation is an immune response in the skin against to the pathogenic microorganisms. However, impaired control of immune reaction can lead to chronic inflammation skin disease1). Skin consists of the epidermis, dermal layers, stratified keratinocytes, and dermis. As the outermost layer of the skin, stratum corneum consists of differentiated keratinocytes. The stratum corneum and skin surface microbial virulence factors including a wide range of molecules produced by pathogenic microorganisms play an important role in keeping the wholeness of the skin2). In this study, anti-inflammatory effects of Raphani semen ethanol extracts(RSE) were investigated through TNF-α and IFN-γ stimulated HaCaT keratinocytes.
There are two main theories regarding atopic dermatitis. Outside-in theory hypothesizes that the intrinsic damage to the skin barrier function caused by disturbed keratinocyte differentiation promotes the entry of allergens and activation of subsequent immune system. Meanwhile, the inside-out theory hypothesizes that the cascade of the immune response induced by Th2 activation in the skin results in the atopic dermatitis phenotype3).
Leaves, seeds, and roots of Raphanus sativus Linné have been used for both edible and medical purposes. Above all, roots were mainly the subject of study, and the research on seeds was relatively small. Raphani semen, the dried ripe seed of Raphanus sativus Linné contains alkaloids, anthraquinon glycosides, terpenoids glycosides, steroids, tannins, carbohydrates, fats, oils, and flavonoids4). It has been reported that Raphani semen had anti-inflammatory and anti-asthmatic property and could be used for the treatment of intestinal inflammatory disorders5,6). Another study revealed that AITC, one of the ingredient of Raphani semen had inflammatory effects in human mast cell7).
However, effects of Raphani semen in HaCaT keratinocytes were not investigated yet. So, this study aims to investigate the effects of RSE at various concentrations in an inflammatory environment induced by TNF-α and IFN-γ stimulated HaCaT keratinocytes through the expression of inflammatory cytokines and transcription factors.
II. Materials and Methods
Dulbecco’s modified Eagle’s medium(DMEM), fetal bovine serum(FBS), penicillin, and streptomycin were purchased from Life Technologies Inc.(Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2 -yl)-2, 5-diphenyltetrazolium bromide(MTT) reagent was imported from Sigma Chemical Co.(St. Louis, MO, USA). Primary antibodies against phospho-IκBα(cat. no. 2859), p-STAT1(cat. no. 9167), extracellular signal-regulated kinase(ERK)(cat. no. 9102), p-STAT6(cat. no. 56554), and STAT6(cat. no. 9362) were obtained from Cell Signaling Technology, Inc.(Danvers, MA, USA). Primary antibodies against IκB-α(cat. no. sc-1643), p-ERK(cat. no. sc-7383), STAT1 (cat. no. sc-271661), periostin(cat. no. sc-398631), and β-actin(cat. no. sc-81178) as well as peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc.(Santa Cruz, CA, USA). Primary antibody against TSLP(cat no. ab188766) was purchased from Abcam, plc.(Cambridge, UK).
The Raphani semen was purchased in Nanum herb(Yeongchen, Gyeongbuk, Korea). Dried and ground Raphani semen(200g) were extracted with 2ℓ 70% EtOH by maceration and the extracts were filtered. The filterate was evaporated in a rotary vacuum evaporator at 40°C, freeze-dried in vacuum using a freeze dryer. Finally, 15.25g of extracts(at a concentration of 50㎍/㎖) were obtained from 214.57g of Raphani semen and stored at 20°C for bioassays.
The HaCaT keratinocytes were provided by Prof. Kyung-Tae Lee(Kyung Hee University, Seoul, Korea). The cells were cultured in DMEM supplemented with 10% FBS, penicillin(100U/㎖), and streptomycin(100㎍/㎖) in a 37℃ and 5% CO2 incubator. Cells were treated with 50㎍/㎖, 100㎍/㎖, and 200㎍/㎖ for 1hr prior to stimulation with TNF-α and IFN-γ(10g/㎖) for the indicated time.
HaCaT keratinocytes were plated at a density of 5×104cells/well in 96 well plates. To determine the appropriate concentration of RSE which has no effect on cell viability, MTT assay was performed at 24hrs following treatment of RSE with various concentrations in HaCaT keratinocytes. Next day, the cells were treated with 50㎕ of MTT(5㎎/㎖) for 4hrs. The formazan precipitate was dissolved in DMSO, and absorbance was measured at 540㎚ using an Epoch microplate spectrometer(BioTek, Winooski, VT, USA).
Lysated cells were suspended in protein extraction solution(PRO-PREP™, Intron Biotechnology, Seongnam, Republic of Korea) and incubated for 20min at 4°C. Cell debris was eliminated by micro-centrifugation, followed by immediate freezing of the supernatant. The protein supernatant concentration was measured by using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc. CA, USA) according to the manufacturer’s instructions. Each protein sample(30㎍) were electro-blotted transferred onto a polyvinylidene fluoride(PVDF) membrane followed by separation using 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Membranes were incubated for 30min with blocking solution(2.5 or 5% skim milk) at room temperature, followed by incubation overnight with a 1:1,000 dilution of primary antibody at 4°C. Membranes were washed three times with Tris-buffered saline/ Tween 20(TBS-T) and incubated with a 1:2,500 dilution of horseradish peroxidase-conjugated secondary antibody for 2hrs at room temperature. Membranes were again washed three times with TBS-T and then developed by enhanced chemiluminescence(GE Healthcare Life Sciences, Chalfont, UK). The chemiluminescent blots were imaged on film. Densitometry analysis of the western blots was done by using ImageJ software(National Institutes of Health, Bethesda, MD). The intensity of the individual bands on western blot analysis was measured by gel analyzer tools of ImageJ and normalized either to β-actin to its total protein. The graphs represent normalized values as fold change over control condition.
The data reported have been expressed as the mean±standard deviation(SD). Data were analyzed using one-way analysis of variance (ANOVA) with Dunnett’s test, and p-values<0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism(Ver. 5.00 for Windows, San Diego, CA, USA).
III. Results
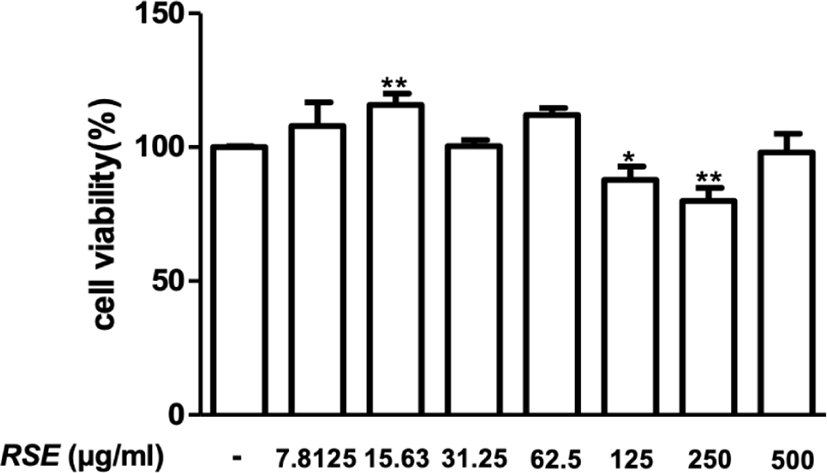
Cytotoxicity of RSE was tested in HaCaT cells to determine the appropriate concentrations. HaCaT cells were treated with different concentration of RSE ranging from 7.8125 to 500 for 24hrs. Since the result showed cytotoxicity at a concentration of 250㎍/㎖, the lower concentrations of 50㎍/㎖, 100㎍/㎖, and 200㎍/㎖ of RSE were set as the sample treatment concentration in this study(Fig. 1).
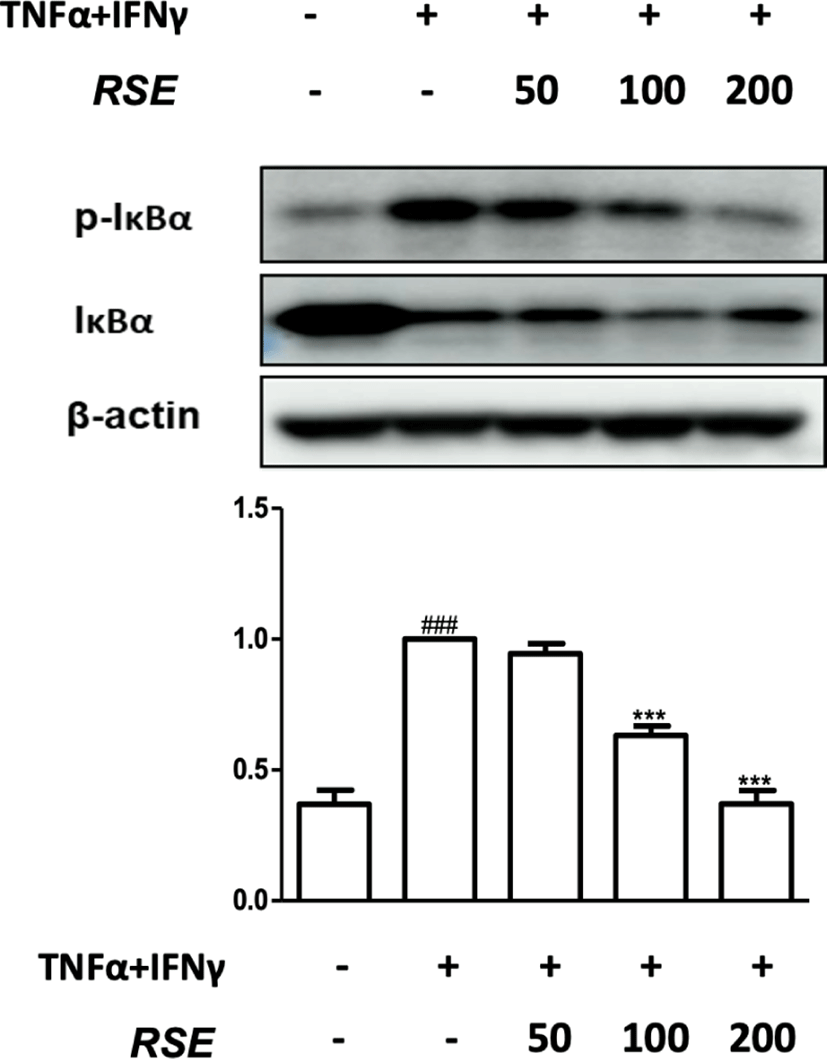
Nuclear factor-κB(NF-κB), a transcription factor, exists in cytoplasm binding to inhibitor of kappa B alpha(IκBα). When IκBα is phosphorylated during an inflammatory reaction, NF-κB translocates to the nucleus, leading to transcription of iNOS, COX-28). Therefore, Western blot assay was performed to confirm the expression of phosphorylation of IκBα(p-IκBα) protein inducing activation of NF-κB. In result, the RSE treatment of 100㎍/㎖ and 200㎍/㎖ significantly suppressed p-IκBα expression in TNF-α and IFN-γ stimulated HaCaT keratinocytes (Fig. 2).
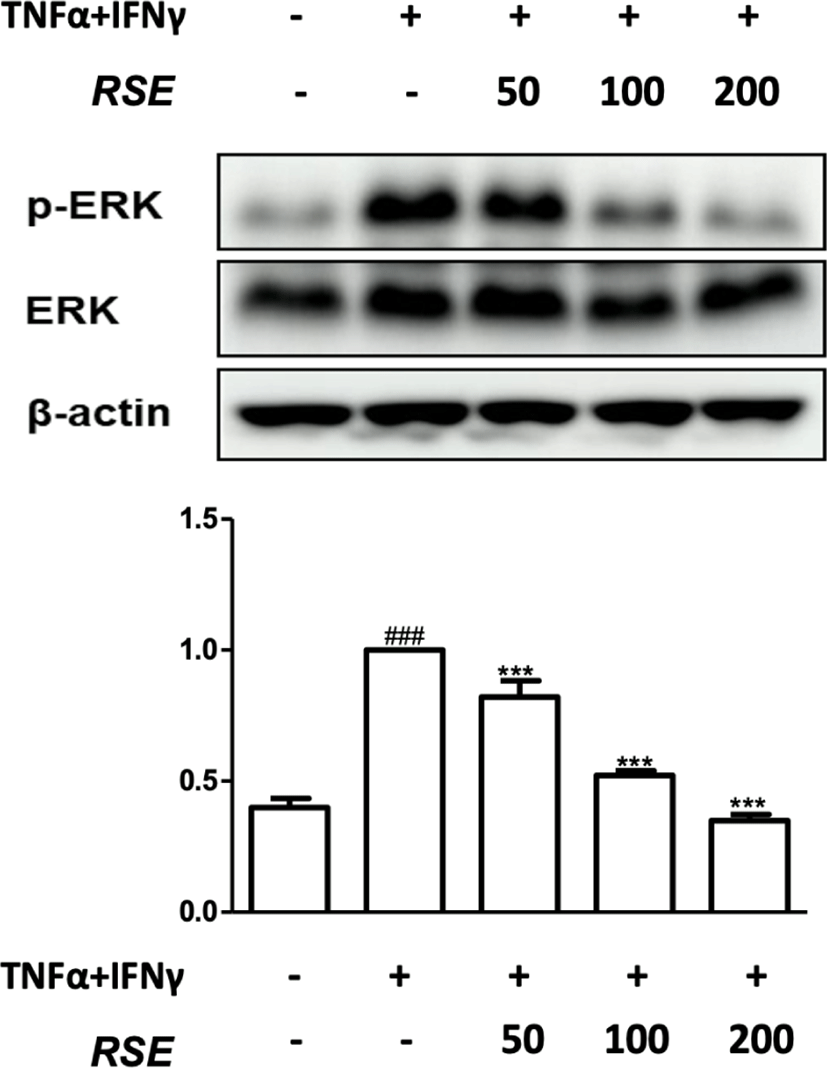
In addition to p-IκBα expression, the phosphorylation of mitogen-activated protein kinases(MAPK) which is involved in the activation of NF-κB was also confirmed. MAPKs consist of ERK, JNK, and p38. When MAPK pathway is activated, it increases cell activity and differentiation, playing an important role in the occurrence of inflammation. ERK activated by IL-1 and TNF-α is associated with signals that induce growth and differentiation of T cell and regulates the synthesis of IL-6, IL-12, IL-23, and TNF-α. Therefore, in order to determine whether the inhibitory mechanism of RSE passes through MAPK pathway, the phosphorylation of ERK was confirmed through western blot analysis9-11).
RSE reduced p-ERK expression in dose dependent manner, indicating that RSE inactivated NF-κB signaling pathway through suppressing the phosphorylation of IκBα and ERK(Fig. 2, 3).
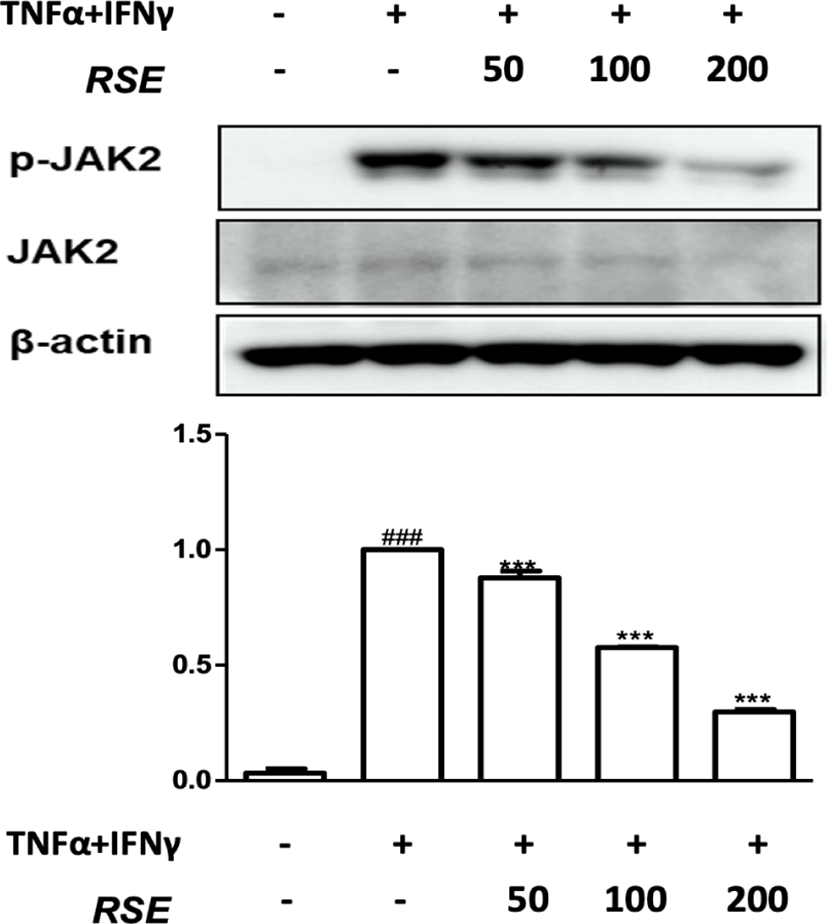
JAK2/STAT1 pathway is one of the inflammatory signals activated by pro-inflammatory stimulation and plays an important role in iNOS expression with NF-κB signals12). To gain insight into the inhibitory mechanism of RSE on the Janus kinases(JAK)/signal transducer and activator of transcription proteins(STAT) signal cascade, we examined the effects of RSE on p-JAK2 expression. The expression levels of p-JAK in the TNF-α and IFN-γ stimulated group significantly increased compared to the control group. In contrast, treatment with 50㎍/㎖, 100㎍/㎖, and 200㎍/㎖ of RSE markedly reduced expression of p-JAK(Fig. 4).
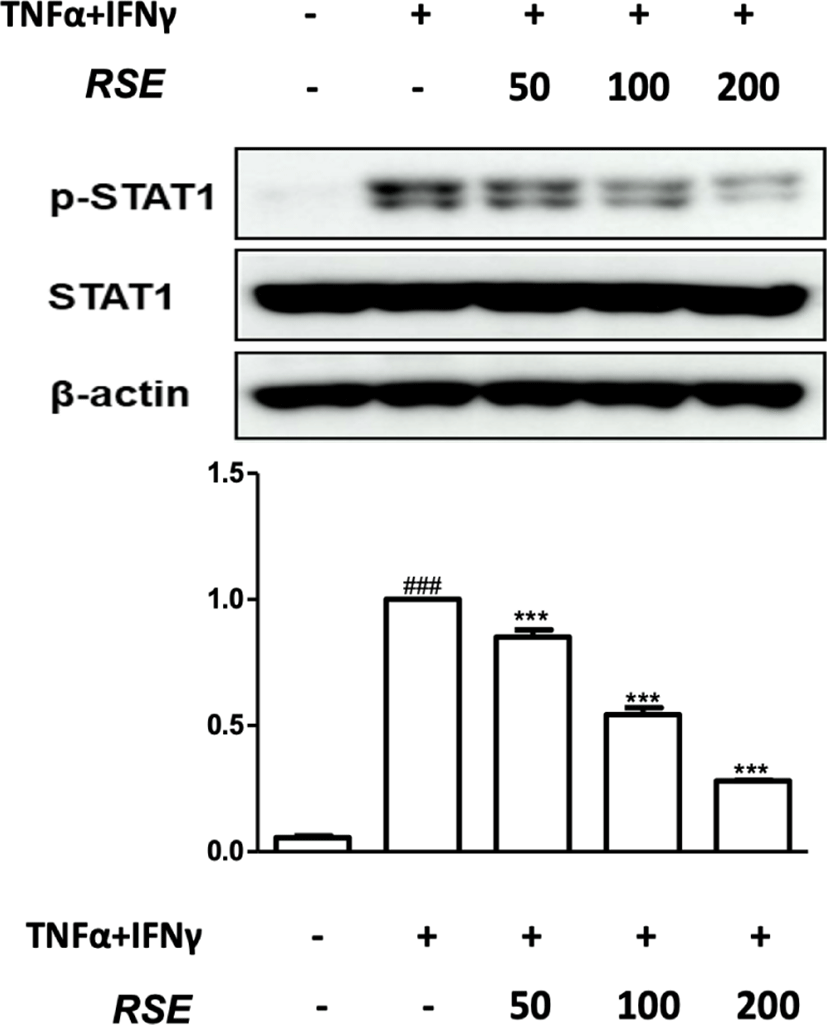
In response to cytokines like TNF-α and IFN-γ, STAT is activated by JAK kinases and MAP kinases13). So, we investigated the inhibitory effects of RSE on STAT1 phosphorylation using a western blot analysis. Results indicate that RSE significantly inhibited STAT1 phosphorylation in dose-dependent manner(Fig. 5).
STAT6 activation is generally induced by stimulating IL-4 receptor. Activated STAT6 translocates from the cytoplasm to the nucleus, inducing target genes including IL-4, IL-5, and IL-1314). At the highest concentration of RSE(200㎍/㎖) significantly suppressed expression of p-STAT6 (Fig. 6).
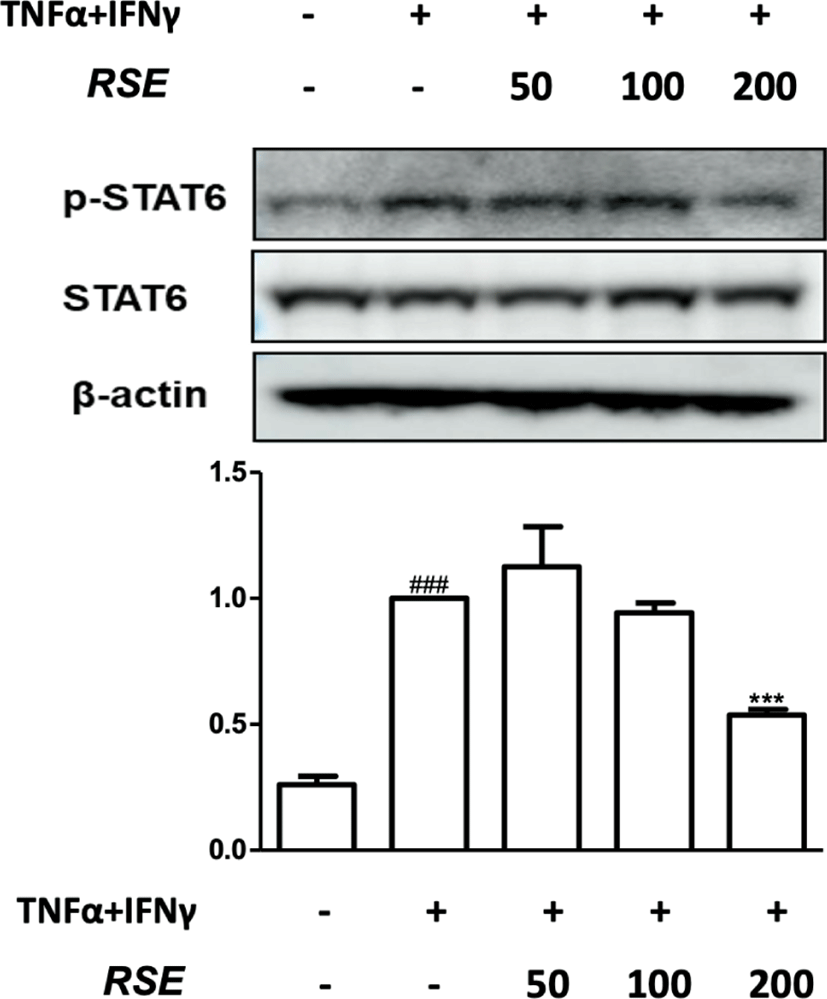
These results demonstrate that RSE inhibits the phosphorylation of STAT1 and STAT6 via JAK2 inactivation in TNF-α and IFN-γ induced inflammatory condition(Fig. 4-6).
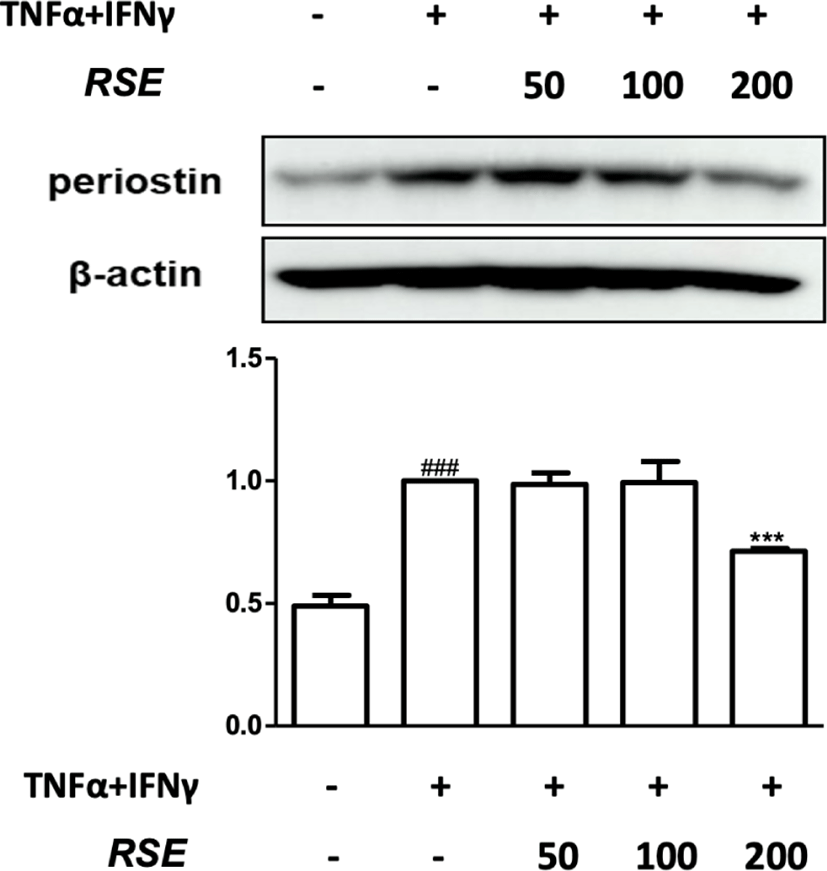
Periostin is well-known to exacerbate allergic dermatitis by inducing the production of Thymic stromal lymphopoietin(TSLP) from keratinocytes, and serum periostin can reflect the severity of atopic dermatitis15). The expression of periostin requires the activation of STAT616).
The 200㎍/㎖ of RSE markedly suppressed expression of periostin(Fig. 8). RSE could decrease the expression of periostin via inhibition of p-STAT6 in HaCaT keratinocytes.
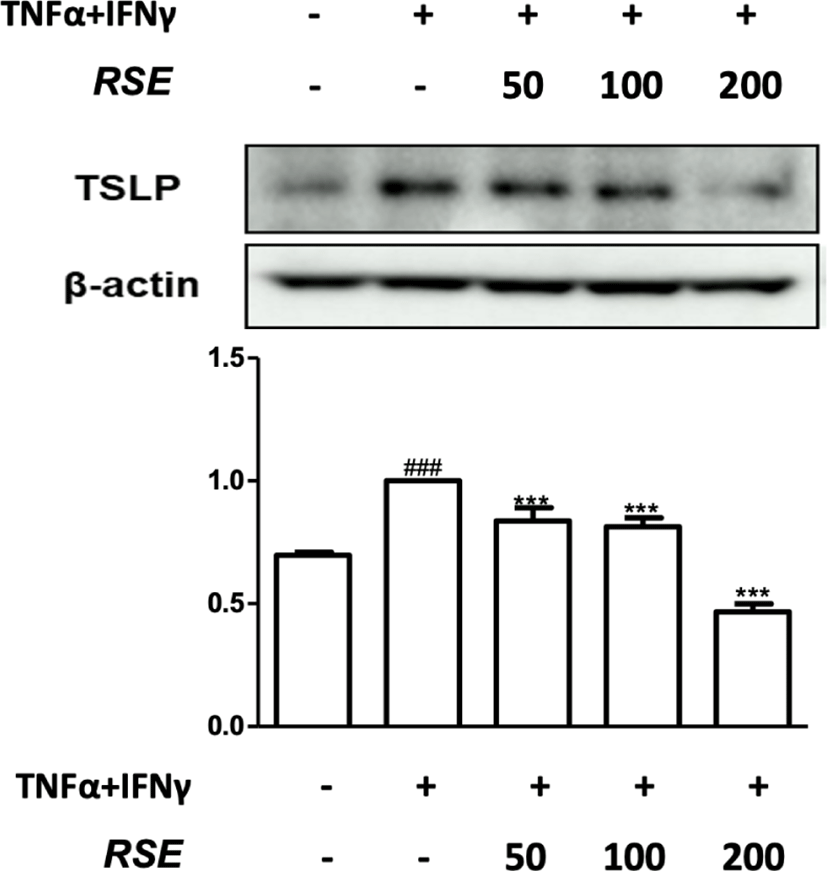
TSLP promotes an inflammatory Th2 response by activation of dendritic cells, playing significant role in atopic dermatitis and other inflammatory disease. In epithelial cells, especially keratinocytes, TSLP was highly increased from atopic dermatitis subjects compared with healthy subjects17).
TNF-α and IFN-γ significantly increased the TSLP expression in HaCaT keratinocytes. However, RSE reduced the TSLP expression(Fig. 8). Production of TSLP is induced by periostin, and TSLP can induce the phosphorylation of STAT615,18). These results suggest that RSE has anti-inflammatory effects by suppressing the production of TSLP via inhibiting the expression p-STAT6 and periostin in HaCaT keratinocytes.
IV. Discussion
The Raphani semen has been traditionally used as laxative, carminative, expectorant, and antitussive in Korea and China19). It has been demonstrated that Raphani semen has anti-bacterial, anti-inflammatory, antioxidant, anti-angiogenic, ACE inhibitory, anti-diabetic, expectorant, diuretic, and laxative effects20-3). The extraction of leaves has diuretic and laxative effects24,25). Also, the extraction of roots could be used for inflammatory disease, urinary problems, hemorrhoids, gastric illnesses, fungal disease, and D-galactosamine induced nephrotoxicity24,26,27). In previous studies, anti-inflammatory functions of Rapahni semen were attributed to the inhibition of NF-κB, p-ERK, and IL-6 in HMC-1 cells and inactivating p38 MAPK and NF-κB pathways in experimental ulcerative colitis models5-7). Thus, in this study we suggest another important anti-inflammatory mechanism of RSE in HaCaT keratinocytes.
Keratinocytes, which account for 95% of epidermal cells, not only produce stratum corneums of the epidermis, but also produce several cytokines and play an important role in inflammatory diseases of the skin28). The human keratinocyte cell line(HaCaT) which is derived from human epidermal keratinocyte line and most similar to human keratinocytes has been used in the in vitro29). In this study, to make similar condition to atopic dermatitis skin we stimulated HaCaT cell with TNF-α and IFN-γ.
Atopic dermatitis inflammation is imbalance of Th1/Th2 immune reaction and IgE induced inflammation. In acute stage of atopic dermatitis Th2 signal dominates, meanwhile in a chronic state, Th2 switch to Th1 signal30-2). Th1 cells produce TNF-α and IFN-γ in chronic atopic dermatitis skin lesions. In HaCaT cells, stimulation of TNF-α and IFN-γ induces Th-2 related chemokines and cytokines like IL-1, IL-6, and IL-833-5).
In order to control the immune and inflammatory reactions, cytokine binds to a specific receptor and then transmits a signal to the target cell nucleus through a signal transmission pathway. Among the signaling pathways of cytokine, NF-κB and JAK/STAT are important transcription factors, and after being produced in ribosomes, they are introduced into the nucleus to promote gene expression, so inhibition of these actions can be crucial in treating atopic dermatitis36).
NF-κB is binding with IκBα in the form of heterodimerization8). However, stimuli induces cells to make IKK complexes composed of IKKα, IKKβ, and IKKγ, also called NF-κB essential modulator(NEMO) get activated37). The activated cells cause phosphorylation and 26 proteasome-mediated degradation of IκB which frees NF-κB. Free NF-κB is translocated into the nucleus and binds to DNA, which stimulates the expression of the target gene, causing inflammatory diseases8).
NF-κB is activated by MAPKs. MAPKs activated by NO, cytokines, EGF, and pathogens induce the production of COX-2, cytokines, and control the cell activation and differentiation. MAPKs consist of three main groups: ERK, p38, and JNK, regulated with each other through associated signaling systems, and plays an important role in signaling systems of immune responses by regulating inflammatory responses in relation to activation of NF-κB9,38). ERK subfamily consists of ERK1, ERK2, and other atypical forms, such as ERK3, ERK4, ERK5, and ERK7. ERK1 and ERK2 are mainly found, so ERK 1/2 pathway has been involved in cell proliferation, growth, differentiation as well as cell migration, cell survival, and transcription39). In this study, RSE suppressed phosphorylation of IκBα and ERK, however phosphorylation of JNK and p38 did not show significant results. Suppressed expression of p-IκBα and p-ERK by RSE indicates that RSE has anti-inflammatory effect through inactivating the NF-κB pathway via inhibition of MAPK pathway(Fig. 2, 3).
Another important transcription factor in signaling pathways of cytokines and growth factors is JAK/STAT pathway. JAK2/STAT1 pathway is one of the inflammatory signals activated by pro-inflammatory stimulation and plays an important role in iNOS expression with NF-κB signals40). JAK group including JAK1, JAK2, JAK3, and TYK2 is an pivotal proteins in signal transduction. STATs are activated by phosphorylation of JAK activation which requires the binding of ligands to cytokines and growth factors receptors12). Especially JAK2 is related with down stream proteins like STATs. So, JAK/STAT has been a major signaling pathway for crucial role in inflammation and cancer41). Induced by IFN-γ, JAK kinases directly and indirectly phosphorylate STAT1 at tyrosine residue at position 701(Tyr701) and serine residue at position 727(Ser727) respectively in a gPI3K- PKC dependent manner42). p-STAT1 converts into nucleus that activates transcription of pro-inflammatory media. A study on murine B cells indicated that the prolonged activation of the transcription factor STAT6 in B cells during chronic allergic inflammation resulted in IgE responses to viral and bacterial antigens resulting from the subsequent microbial infection that follows the onset of atopic dermatitis. These IgE responses stimulated further activation of mast cells resulting in inflammation43). It was observed that RSE inhibited STAT1 and STAT6 phospholyation via JAK2 inactivation in TNF-α and IFN-γ induced inflammatory condition(Fig. 4-6).
Through JAK/STAT pathway, keratinocytes secrete periostin in response to cytokine TSLP44). It was confirmed that atopic dermatitis did not occur when an antibody preventing the binding of periostin and receptor was administered to experimental mice and then house dust mite extract was applied to the mouse skin16). Also, according to the results of the recent study, serum periostin was significantly increased in children with exogenous atopic dermatitis, and also found to be correlated with SCORAD index and peripheral blood eosinophils. It means that periostin could be an indicator for understanding the pathological mechanism, degree of symptoms, and prognosis of atopic dermatitis45). TSLP acts as an initial stimulation factor for the skin immune response. When the skin is damaged and exposed to external antigens, TSLP is secreted from keratinocytes, inducing the secretion of inflammatory cytokines including IL-4, IL-13, IL-5, and TNF-α, but producing decreased levels of IL-10 and IFN-γ. IL-10 and IFN-γ down-regulate Th2 inflammation, especially IFN-γ distorts the Th1/Th2 balance in differentiating T cells17,46).
Allergens such as house dust mite initiate inflammatory responses, then lymphocytes increase the expression of periostin in dermis. So periostin is acculmulated in dermis, resulting in up-regulating proliferation of TSLP-producing keratinocytes as well as promoting induction of TSLP expression which contributes to the accelerating Th2 inflammation. It means that periostin contributes to chronic inflammation and deteriorates allergic dermatitis by allowing immune cells to interact with non-immune cells15,16). In the present study, RSE suppressed the expression of p-STAT6, periostin, and TSLP, suggesting that RSE could stop the loop involving the periostin(i.e., p-STAT6 → periostin → TSLP → p-STAT6 → periostin)(Fig. 6-8).
This study verified for the first time that RSE inhibited NF-κB and JAK/STAT pathway in inflammatory environment induced by TNF-α and IFN-γ stimulated HaCaT keratinocytes. Also, the possibility of RSE as an external preparation in inflammatory skin diseases such as atopic dermatitis was proved in this study.
V. Conclusions
This study demonstrated mechanism of anti-inflammatory effects of RSE in TNF-α and IFN-γ stimulated HaCaT keratinocytes.
-
HaCaT cells were treated with RSE, and RSE did not show cytotoxicity below 250㎍/㎖, so concentrations of 50㎍/㎖, 100㎍/㎖, and 200㎍/㎖ of RSE were used in this study.
-
RSE suppressed the phosphorylation of IκBα and ERK in TNF-α and IFN-γ stimulated HaCaT keratinocytes.
-
RSE suppressed the phosphorylation of JAK2, STAT1, and STAT6 in TNF-α and IFN-γ stimulated HaCaT keratinocytes.
-
RSE inhibited the expression of periostin and TSLP in TNF-α and IFN-γ stimulated HaCaT keratinocytes.
These results showed that RSE could be used as a treatment for inflammatory disease through inhibiting NF-κB, MAPK and JAK/STAT pathways and suppressing the expression of periostin and TSLP.






