I. Introduction
Allergic diseases, also known as immune disorders, commonly referred to as immediate or type I hypersensitivity reactions with IgE. Crosslinking of the high affinity IgE receptor(FcεRI) is an essential role in the IgE-mediated allergic reaction1). Molecular interaction between IgE and allergen on mast cells or basophils leads to allergic reactions inducing the release of inflammatory cytokines resulting in the inflammation leading to clinical symptoms in the target organ2).
Asthma is a chronic inflammatory disease of the airways characterized by reduced respiratory function and increased infiltration of leukocytes, especially eosinophils, and it leads to increased hyperresponsiveness, bronchoconstriction and mucus production3). Both eosinophils and T helper(Th) 2 lymphocytes play pathogenic roles associated with increased infiltration of inflammatory cells and mucus secretion in asthma4). Eosinophils commonly linked with allergic inflammation act as effector cells by secretion of cytotoxic granule proteins in the pathogenesis of asthma5). Airway eosinophils with inflammatory cytokines such as interleukin-13(IL-13) may finally result in airway hyperresponsiveness(AHR) in asthma6).
Perilla leaves(Perilla frutescens; Labiatae, PF), the roots of Peucedanum praeruptorum (PP) and the root of Scutellaria baicalensis(SB) are plants used for traditional medicines in East Asia. Considerable attention has recently been devoted to the anti-allergic, anti-inflammatory, and antitumor-promoting substances in perilla plants7). PP is used in alimentary and bronchial disorders and chest pains8). SB is used as an anti-inflammatory drug and smooth muscle relaxant9). Recently, it is focused that herbal medicine is effective for asthma medications for the treatment of adult patients with moderate and severe allergic asthma10). Therefore, complementary medicine for asthma treatment has been newly developed. Also, combinations of herbal extracts are more effective because many herbal mixtures have synergistic effects and can neutralize toxic or adverse effects of their individual constituent herbs11,12).
In the present study, we first investigated whether PF, PP and SB exerts anti-inflammatory effects in RAW 264.7 cells and inhibits mast cell degranulation in RBL-2H3 cells. And then, we evaluated the effects of five different ratios of mixture of PF, PP and SB(PS1-5) on OVA-induced asthma in BALB/c mice. These experimental results indicated that a mixture was most effective when the rate of PF, PP and PP was 2:1:1. An optimized combination of PS(KM1701) was thus chosen in this study. We next investigated the effects of KM1701 on mast cell degranulation in vitro and eosinophils infiltration in vivo.
II. Materials and methods
The dried leaves of PF, root of PP and SB were purchased from the Kwangmyungdang Medicinal Herbs Market(Ulsan, Republic of Korea). Raw materials were stored in a low temperature warehouse(4±2°C) until use. The extraction was performed as follow: 100g of raw materials(PS1 PF:PP:SB=1:1:1, PS2 PF:PP:SB=2:1:1, PS3 PF:PP:SB=3:1:1, PS4 PF:PP:SB=2:1:2, PS5 PF:PP:SB=2:2:1) was extracted by refluxing 80°C, 50%(v/v) ethanol(Duksan Pharmaceutical Co. Ltd., Repulic of Korea) for 3h two times. Alcoholic extract was filtered through a 0.22㎛ filter and evaporated at 40°C and then freeze-dried. The powder was kept at 4°C before use.
RAW 264.7 mouse macrophage cells and RBL-2H3 rat basophilic leukemia cells were purchased from the American Type Culture Collection(ATCC; Manassas, VA, USA) and cultured in DMEM supplemented with 10% FBS, 100 U/㎖ penicillin and 100㎍/㎖ streptomycin at 37°C in a humidified 5% CO2 atmosphere. The cytotoxicity on RAW 264.7 cells was measured with an 3-(4,5-Dimethylthiazol-yl)-diphenyl tetrazolium bromide(MTT, Sigma, St. Louis, MO, USA)-based colorimetric assay. All experiments were performed 24h after RAW 264. 7 cells seeding in 96-well plates. In brief, cells were treated with indicated concentrations of extracts for 24h. MTT solution was added at the end of the treatment to the cell culture media at 0.5㎎/㎖ final concentration and incubated for 2h at 37°C in the dark. The absorbance at 595㎚ was determined with a microplate reader(Infinite F200 PRO, Tecan Trading AG, Switzerland).
After RAW 264.7 cells(1.0×104cells/well) were seeded in a 96-well plate, cells were pretreated with extract(12.5, 25, 50, 100㎍/㎖) for 1h prior to stimulation with LPS(1㎍/㎖) for 23h. The NO concentrations were assessed using Griess reagent assay kit(G2930, Promega Korea, Ltd., Korea).
The β-hexosaminidase release inhibition assay using RBL-2H3 cells was performed as previously described13). For the β-hexosaminidase inhibition assay, RBL-2H3 cells were seeded onto 24-well plates at 2.0×104cells/well in 400㎕ of medium. The cells were incubated for 24h at 37°C and sensitized with 0.5㎍/㎖ anti-dinitrophenyl(DNP)-IgE, then washed twice with PIPES buffer(25mM PIPES, 119nM NaCl, 5mM KCl, 5.6mM glucose, 0.4mM MgCl2, 1mM CaCl2, 40mM NaOH, 0.1% BSA, pH7.2) to eliminate free IgE. After washing, the cells were preincubated with either 400㎕ PIPES buffer containing extract(final concentration 100㎍/㎖, respectively) at 37°C for 10 min. RBL-2H3 cells were exposed to 100㎍/㎖ DNP-Human Serum Albumin(HAS) in PBS, followed by incubation at 37°C for 1h. 25㎕ cell culture supernatant were incubated with an equal volume of 5mM substrate solution(5mM p-nitrophenyl-N-acetyl-β-D-glucosaminide dissolved in 0.2M sodium citrate buffer, pH 4.5) at 37°C for 1.5h. Then, the enzyme reaction was terminated by adding 200㎕ of stop solution(0.1M Na2CO3/NaHCO3, pH 10.0) and the absorbance was measured at 405㎚ with a microplate reader.
Six-week-old female BALB/c mice were obtained from Daehan Biolink Co. Ltd.(Seoul, Korea) and kept in an air-conditioned room at room temperature(about 22±1°C) and a humidity of about 55±10%. Animal treatment and maintenance were carried out in accordance with the Principle of Laboratory Animal Care(NIH publication number 85-23, revised 1985) and the Animal Care and Use Guidelines of Kolmar Korea, Sejong, Korea(Approval No. 17-NP-AST-002-P/Korea Kolmar Animal Test Ethics Committee).
The allergic lung inflammation of the mice was induced by ovalbumin(OVA, Sigma Chemical Co., St. Louis, MO, USA) using the established protocol in a previous study 14). The normal group was sensitized and challenged with PBS without OVA and drug treatment. Female BALB/c mice were sensitized by intraperitoneal injections of 20㎍ OVA and 2㎎ imject® Alum(77161, Termo scientific Korea Ltd., Korea) suspended in 0.1㎖ saline on days 0, 7 and 14. On days 24–27, animals were challenged with 1% OVA aerosol for 50min. Extract(400㎎/㎏/day, PO), montelukast(Sigma Chemical Co., St. Louis, MO, USA)(50㎎/㎏/day, PO) or vehicle(0.5% Carboxylmethyl cellulose, PO) was given 2h before each OVA aerosol challenge. Animal experiments were performed according to the Institutional Guidelines for Animal Care and Use of Kolmar Korea Co., Ltd.
All mice were sacrificed on day 28, and tracheotomies were performed. After ice-cold PBS(1㎖) was instilled into the lungs, bronchoalveolar lavage fluid(BALF) was obtained via tracheal cannulation15). Total inflammatory cell numbers were assessed by counting cells in at least five squares of a hemocytometer, after exclusion of dead cells staining with trypan blue. 100㎕ BALF was loaded onto a slide and centrifuged(200 Xg, 4°C, 10min) to fix the cells onto the slide using a cytospin machine(Hanil Science Industrial, Korea). Cell pellets was suspended in 1㎖ of PBS, and 100㎕ of each solution was spun onto a slide. After slides were dried, cells were fixed and stained using Diff-Quick Stain reagents(38721, Sysmex Corporation, Japan) according to the manufacturer's instructions.
The collected blood should be centrifuged immediately to separate the blood cells from the plasma fraction. After centrifugation, the fibrin clot forms in the upper layer; this clot should be squeezed gently using a pair of sterile forceps and subsequently centrifuged to completely separate the blood cells from the serum. Serum was collected and stored at -70°C. Total IgE and OVA-specific IgE were measured by anti-ovalbumin IgE mouse ELISA kit(500840, Cayman Chemical, MI, USA) according to the manufacturer’s instructions.
All statistical parameters were calculated using Graphpad Prism 5.0 software(GraphPad Software, Inc., La Jolla, CA, USA). Value were expressed as the mean±standard error of the mean(SEM). NO, MTT and β-hexosaminidase assays were evaluated by one-way analysis of variance(ANOVA) analysis followed by the Tukey’s post hoc test. Animal studies were evaluated by the Student’s t-test. Differences with p-value less than 0.05 were considered statistically significant.
III. Results
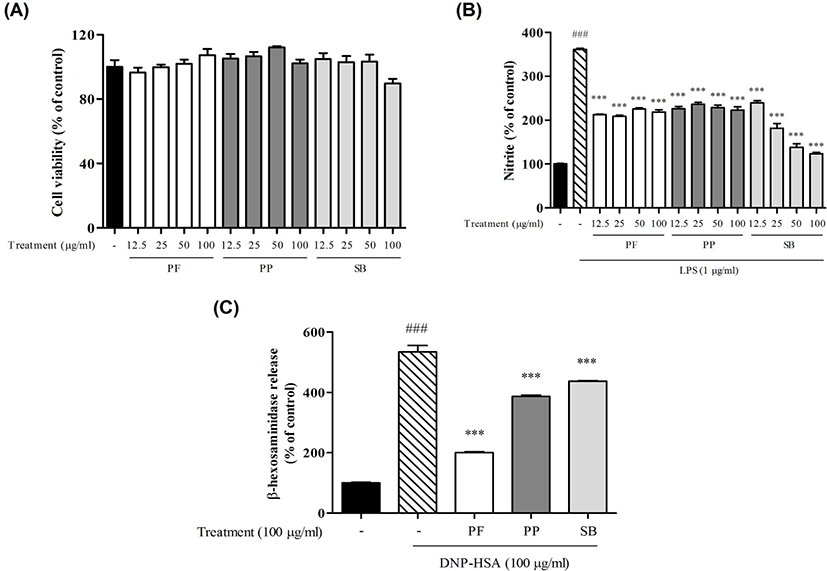
The cytotoxic effects of PF, PP and SB in RAW 264.7 cells were measured by the MTT assay. PF, PP and SB did not significantly affect cell viabilities at concentrations of 12.5, 25, 50 and 100㎍/㎖(Fig. 1A). To examine whether PF, PP and SB inhibit the expression of the NO, we performed Griess reagent assay in RAW 264.7 cells. PF, PP and SB significantly inhibited NO production at concentrations of 12.5, 25, 50 and 100㎍/㎖(Fig. 1B). And then, we examined whether degranulation of mast cells was decreased(Fig. 1B, 1C). PF, PP and SB significantly decreased the release of beta-hexosaminidase.
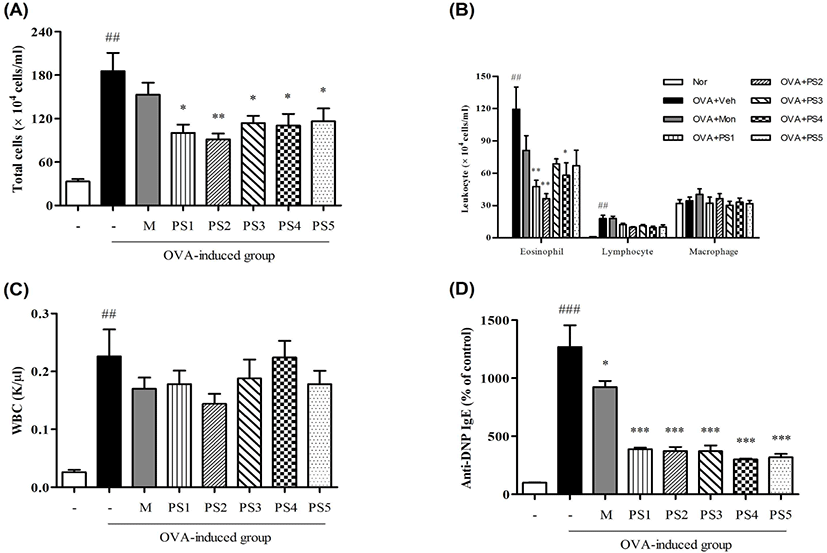
We induced asthma in BALB/c mice by ovalbumin sensitization and challenge. And then, mice were treated with PS1-5(400㎎/㎏/day) and examined the alternation of total cells in the BAL fluid and the level of anti-DNP IgE in serum in order to estimate the effects of PS1-5 on asthma. All treatment groups significantly reduced the numbers of total inflammatory cells, and PS2 is most reduced the number of increased eosinophils(Fig. 2A, 2B). Using hematology analyzer, PS2 is most reduced the number of WBC. And this result is similar to precedents(Fig. 2C). We investigated the effects of PS1-5 to inhibit the production of anaphylactic IgE. Treatments of PS1-5 significantly reduced the production of IgE(Fig. 2D).
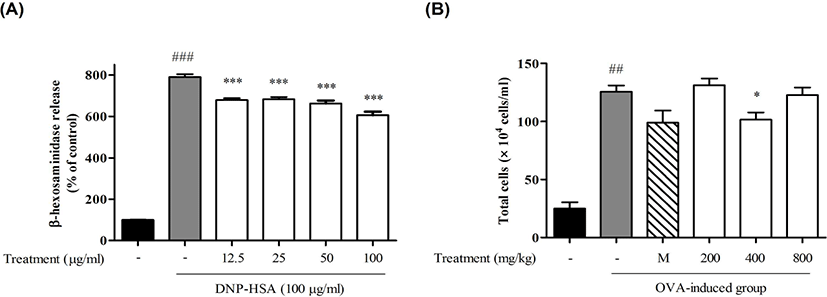
We optimized combination rate 2:1:1 mixture of PF, PP and SB(KM1701), because the present results indicated that KM1701 was most effective. To optimize dose of KM1701, We evaluated inhibitory effects of KM1701 on mast cell degranulation and infiltration of immune cells related asthma in BALB/c mice by ovalbumin sensitization and challenge. KM1701 significantly decreased the release of beta-hexosaminidase(Fig. 3A) in dose-dependent manner and the number of immune cells related asthma cells in BALF(Fig. 3B).
IV. Discussion
In the present study, PF, PP and SB effectively reduced NO production in LPS-induced RAW 264.7 cells and the release of beta-hexosaminidase in RBL-2H3 cells. Therefore, these medicinal plants had potent inhibitory effects on mast cell degranulation and anti-inflammatory properties. The suppressive effects of PS on airway inflammation were noted by a marked decrease in recruitment of inflammatory cells into BALF and in levels of IgE in serum. These results demonstrated the potent anti-inflammatory effect of PS in OVA-challenged mice.
Macrophage has essential roles in the initiation and maintenance of inflammation. LPS-stimulated macrophage results in the production of NO, playing a key role in the pathogenesis of inflammation. We investigated whether PF, PP and SB show potent inflammatory effects in LPS-induced RAW 264.7 cells. The results showed that all extracts inhibit the NO production. RBL-2H3 cells, rat basophilic leukemia cells, have properties of mucosal-type mast cells and express several FcεRI on the membrane surface. RBL-2H3 cells have been widely used for mast cell degranulation through the antigen-induced aggregation of FcεRI16). The β-hexosaminidase is usually released with histamine when mast cells are immunologically activated, during the cross-linking of FcεRIs17). Therefore, to evaluate antiallergic activities of either compounds or herbal extracts, β-hexosaminidase is used as a marker of mast cell degranulation16,18,19). We investigated whether PF, PP and SB could inhibit mast cell degranulation in RBL-2H3 cells. The results showed that all extracts inhibit mast cell degranulation.
Asthma, an inflammatory disease of the lungs and airways, is characterized by increased infiltration of leukocytes, especially eosinophils, and reduced airway function. The inflammation causes airway hyperresponsiveness, bronchoconstriction, and mucus production15). Eosinophils are the major cell types that can be recruited to sites of immunological or inflammatory responses20). The increased numbers of eosinophils in BALF is a hallmark of asthma. In the present study, PS-treated mice had significantly reduced lung eosinophilia in BALF compared with PBS-treated mice. IgE has been closely related with Th2-type responses. Treatment of PS to OVA sensitized/challenged mice significantly decreased serum IgE levels. Therefore, these results indicate that oral administration of PS inhibited IgE production and eosinophilic infiltration.
V. Conclusion
In the present study, PF, PP and SB significantly inhibited the production of NO and the release of beta-hexosaminidase in LPS-activated RAW 264.7 cells and IgE-sensitized RBL-2H3 cells, respectively. We also demonstrated for the first time that PS attenuates the production of IgE and eosinophils infiltration in OVA-sensitized/challenged allergic asthma model mice. These results suggest that PS can abrogate the symptoms of asthma via anti-inflammatory activities and inhibitory effects on mast cell degranulation in vitro and in vivo. Our findings suggest that PS may be promising candidates for the development of a potent therapeutic agent for allergic asthma.






